Globally, the global regenerative medicine market is estimated to reach $39,325 million by 2023, registering a CAGR of 32.2% from 2017 to 2023. The most significant number of marketed cell therapy products is used for the treatment of notably non-healing wounds/skin (46%) and muscular-skeletal injuries (35%). This trend will change as more and stem cell therapy products for cancer and heart disease complete their clinical trials and are approved for market release.
Adult stem cell leads the market due to low contamination during sub-culture and expansion, relatively small labor production and compatibility with the human body. Just the Induced pluripotent stem cells (IPSc’s) are expected to report revenue of over USD 4.5 billion by 2020, on account of the similar nature of its origin. With the continued growth of medical tourism hubs like India, Singapore, and Thailand, Asia is expected to maintain its place as the epicenter of stem cell research and therapy.
The Taiwan government aims to transform Taiwan into a regenerative medicine hub in Asia and to establish a robust regulatory and R& D environment for such procedures. According to reports, six-cell therapies recently approved by the government to treat medical issues, including cancer, degenerative joint disease, and skin wounds, are expected to be available at permitted medical centers.
Some of the significant challenges faced by Taiwan regenerative medicine companies are the higher cost of R&D for the development of RM products, clinical trial approvals, and other regulatory hindrances.
There are about 20 companies in Taiwan that are various stages of clinical development working on different areas of ATMP’s such as autologous DC’s, ADSCs, SMSC’s, DPC, DF, NK, allogenic DF, SMSC, ADSC, BMSCs CAR-T, Neo-T, and auto-activated T cell therapies. These companies are working on various cancers, osteoarthritis, stroke, kidney diseases, and so on.
日期 & 時間
2020.09.01 ~ 2020.09.03
地點 & 地址
主辦單位
聯絡資訊



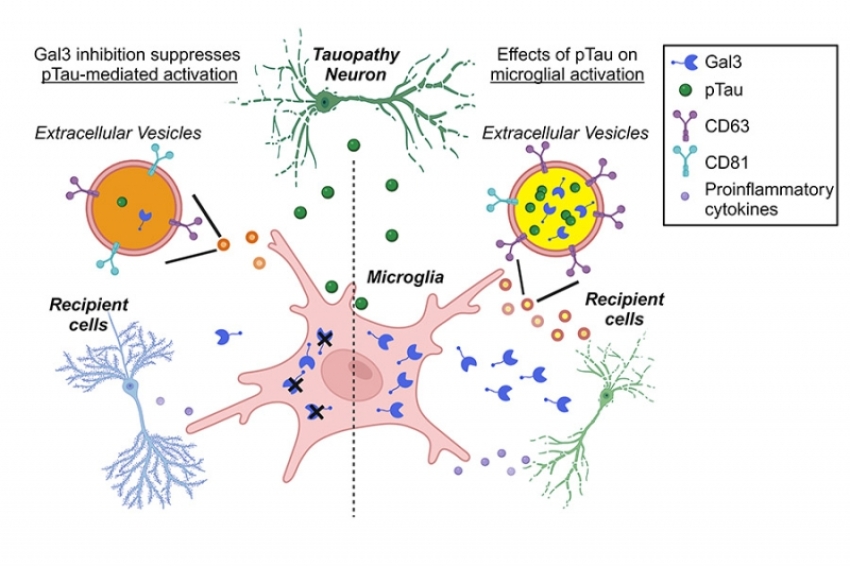






![衛福部核可之細胞治療執行單位 [持續更新]](https://biomaptw.com/media/k2/items/cache/753a82091bdf93df272697e1f26229c2_XL.jpg)